Stanford researchers have developed a microchip that could diagnose type I diabetes quicker than conventional methods, for only a fraction of the cost.
Physicians used to diagnose diabetes in a relatively straightforward manner considering the age of patients. Children were more likely to have type 1 diabetes (T1D), an autoimmune disease, whereas older and overweight patients were presumed to have type 2 diabetes (T2D), a condition caused by insulin resistance. But due to an epidemic of obesity and other unexplained factors, the distinction between the two types is blurring. Today, more children are being diagnosed with type 2 diabetes, and there are more adults with type 1 diabetes than in the past.
Current lab methods like lateral-flow assays, ELISA, and radioimmunoassay are able to distinguish between the two types by detecting autoantibody biomarkers of T1D. But these methods are expensive, cumbersome and usually take days to yield results.
Now, researchers at Stanford University say they have developed a new diagnostic tool that is cheaper, simpler, and just as effective as conventional lab standards. The palm-sized microchip can analyze a few drops of blood from a finger prick instead of full blood draws. Results are in after just an hour instead of waiting for days or weeks in the case of other tests. Moreover, the device costs only around $20 to produce, much cheaper than the hundreds of dollars needed for lab tests that detect those autoantibodies.
In the study published recently in the journal Nature Medicine, the researchers described their new device as a “plasmonic gold chip for near-infrared fluorescence–enhanced (NIR-FE) detection of islet cell–targeting autoantibodies” with “high sensitivity and specificity for the diagnosis of T1D and can be used to discover previously unknown biomarkers of T1D.”
The glass plates forming the base of each microchip are coated with nanogold particles that identify three types of autoantibodies simultaneously by giving off intensified fluorescent signals. The devices were tested on 39 individuals with new onset diabetes and non-diabetic controls. The new tool was found equal in sensitivity (100 percent) and specificity (85 percent) to conventional radioimmunoassay in detecting T1D, according to a Medscape article.
Detection of one antibody means a person has an increased risk of developing diabetes, while having two or more antibodies signifies more than a 90 percent risk. The researchers said that the tool could not only diagnose patients, but their at-risk relatives as well.
“That’s one of the best crystal balls that exists in medicine,” Dr. Brian Feldman, lead author and assistant professor of pediatric endocrinology at Stanford University School of Medicine, told the San Francisco Chronicle. “We feel like this now is not only going to lead to better diagnostics and access to next-generation therapies, but allow people to do trials or intervention and provide a risk assessment of who will develop diabetes in the future.”
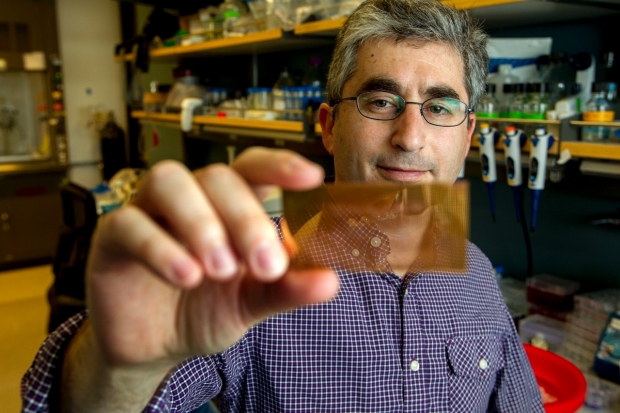
"There is great potential to capture people before they develop the disease, and prevent diabetes or prevent its complications by starting therapy early,” Feldman added. “But the old test was prohibitive for that type of thinking because it was so costly and time-consuming.”
Because of the cost-effectiveness of the microchip device, the researchers anticipate its use in large diabetes screening programs around the world. Physicians and even other health workers with only basic training can make use of such a portable diagnostic kit to quickly identify those at risk and start timely management at the early onset of diabetes.
“We need less than 2 microliters — just poking the finger and using the blood that way. It’s just more acceptable to the patient populations and allows us to dream of moving this to primary care offices or point-of-care situations and someday — not yet — to have the pediatrician know in his or her office whether this is type 1 or type 2,” Feldman said in an interview with the Washington Business Journal. “They don’t need to send the patient to a lab.”
The novel device could provide a tremendous boost in efforts to fight diabetes. According to the Juvenile Diabetes Research Foundation, the prevalence of Type 1 diabetes in Americans younger than 20 jumped 23 percent between 2001 and 2009. This is part of an overall rise of type 1 diabetes worldwide. But the increase is “steeper in the Middle East than Europe or the USA,” according to The Lancet, citing genetic and lifestyle factors. In the United Arab Emirates alone, the number of children diagnosed with type 1 diabetes has doubled since 2000.
Feldman and colleagues will license the technology from Stanford and build their startup called IGI Stat. The project follows the footsteps of fellow Stanford researcher Elizabeth Holmes, who formed her own company Theranos, Inc. which offers diagnostic tools with near-instantaneous lab results using just a few drops of blood.
Log in or register for FREE for full access to ALL site features
As a member of the nuviun community, you can benefit from:
- 24/7 unlimited access to the content library
- Full access to the company and people directories
- Unlimited discussion and commenting privileges
- Your own searchable professional profile



.jpg)







.jpg)
